
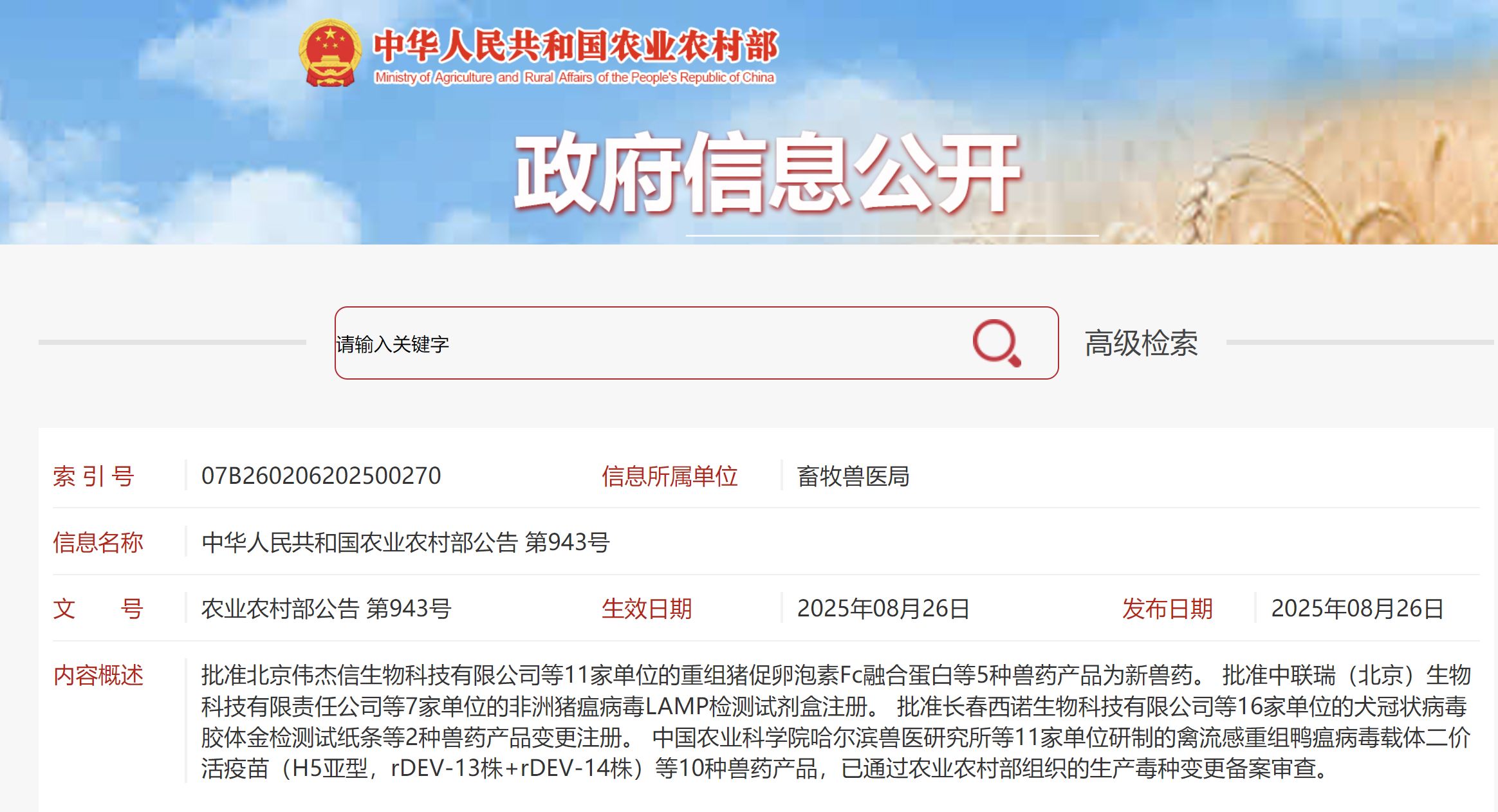
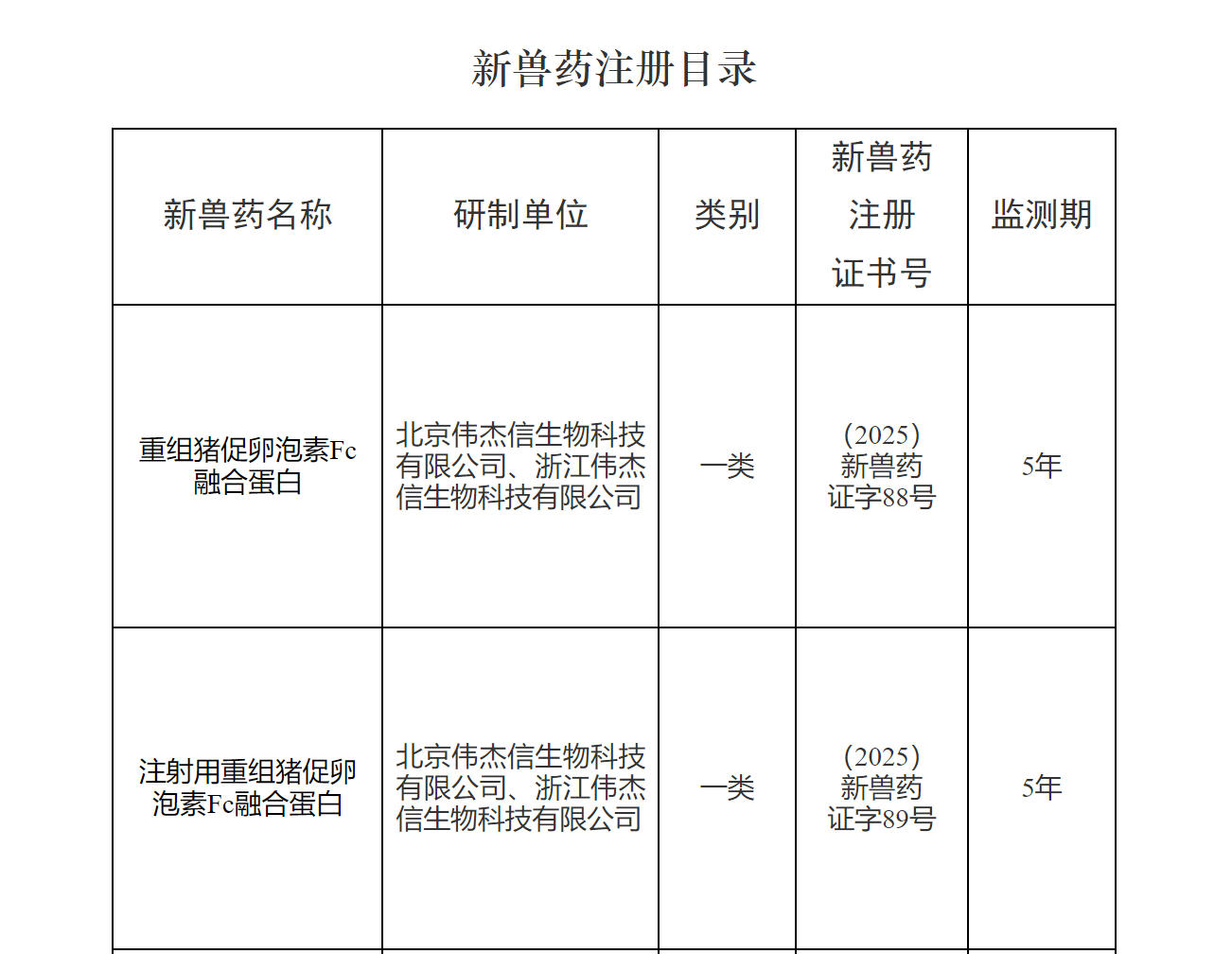
On August 26, 2025, a historic breakthrough was achieved in China's innovative veterinary drug sector. VJTBio's self-developed innovative drug for livestock reproduction – Recombinant Porcine Follitropin Fc Fusion Protein – was officially approved by China's Ministry of Agriculture and Rural Affairs, receiving the National Class I New Veterinary Drug Certificate. This marks China's first innovative biological veterinary drug with fully independent intellectual property rights and a global patent portfolio. As a product for batch reproduction management under new global farming models, it offers comprehensive advantages of eco-friendly, safe, efficient, and "residue-free." The approval of this new drug not only signifies China's leading position in animal reproduction biotechnology but also resolves critical "bottleneck" challenges in reproduction and breeding as well as intensive, large-scale farming. It provides a "Chinese Solution" for ensuring global agricultural security and promoting the healthy development of the animal husbandry industry, heralding the arrival of the Efficient Breeding 3.0 Era.
Nine Years of Hard Work Culminates in a Transformative Achievement
VJTBio operates in the global innovative veterinary drug market, valued at hundreds of billions, and is a biological pharmaceutical company integrated across R&D, production, and sales in the animal health sector. VJTBio has consistently adhered to its philosophy of "Caring for Animals, Committed to Humanity; Reverence for Life, Devoted to Drug Development". It advocates for green and safe farming practices, developing high-quality, "residue-free" protein drugs to meet the high-quality development needs of large-scale, intensive farming enterprises, thereby providing the public with safer meat, eggs, and milk. Concurrently, VJTBio develops antibody drugs to treat pet diseases, aiming to bring more joy and emotional comfort to hundreds of millions of pet owners and enhance quality of life. Over nine years, VJTBio’s R&D team has tirelessly pursued innovation. Every step of the journey—from the meticulous accumulation of laboratory data, through repeated optimizations during the pilot-scale phase, to the thorough validation in clinical trials—embodies the dedication and perseverance of the VJTBio team. Amidst these challenging R&D efforts, the company consistently increased investment, establishing China's first CHO cell-based GMP production line for animal recombinant protein and antibody drugs. It has built a comprehensive technological system spanning molecular design, process development, and commercial production, providing a solid foundation for innovative drug development.
Precise Reproduction Management Empowers Industry Upgrade
This product represents a key breakthrough as the first high-efficiency CHO cell expression technology platform in the veterinary drug field. Its development and production strictly adhere to international biological drug standards, ensuring safety, efficacy, and controlled quality. At the same time, the VJTBio technical team has built comprehensive, product-centric solutions addressing core issues such as reproduction management, precision farm management, and biosecurity. The integrated application of the product and technology effectively addresses a range of industry challenges, such as resolving second-litter syndrome, improving the return-to-estrus and conception rates of first-parity sows, increasing batch utilization efficiency, and managing estrus issues in overdue sows.
Clinical trial data demonstrates that the use of this product leads to breakthrough improvements in litters per sow per year and PSY (Piglets Weaned per Sow per Year). Furthermore, as a homologous recombinant protein drug, it possesses inherent natural and safe characteristics, offering significant advantages in terms of safety. The product can be administered multiple times and repeatedly, meeting the demands of modern large-scale farming and providing a more efficient and safer reproduction management solution for the breeding industry. Proven by trial results, it substantially enhances reproductive efficiency, effectively reduces farming costs, and significantly increases farming profits.
Resolving Critical "Bottleneck" Challenges, Safeguarding Industry Security
The successful development and localization of the innovative protein drug for animal reproduction by VJTBio has achieved technological self-sufficiency. With its comprehensive performance metrics, it now benefits various types of breeders across China. The drug has already received authorization in countries including the United States, Russia, Australia, New Zealand, and Mexico, and has reached cooperation intentions with major animal husbandry nations in Southeast Asia and South America. In the realm of green farming, as the world's first long-acting "residue-free" animal protein drug in the reproduction field, it facilitates the safe and efficient production for tens of millions of breeding sows, effectively resolving the critical "bottleneck" challenges in China's reproduction regulation drug sector.
Looking ahead, VJTBio will consistently and steadily increase R&D investment, deepen its focus on the main track of innovative drug development, and build a comprehensive product portfolio covering animal reproduction, disease prevention and control, and pet health. At the same time, leveraging its self-developed platform for innovative veterinary drug development, the company will deepen cooperation with domestic and international universities and research institutions, continuously introduce and cultivate innovative talents, and fully leverage the pivotal role of new quality productivity in the animal health sector. This will enable Chinese innovative drugs to secure a significant position in the global animal health market, contributing more Chinese wisdom and strength to safeguarding global life and health, enhancing animal welfare, and promoting sustainable agricultural development.